How do Lithium-ion Batteries work?
Inside a Lithium-ion battery there is a negative and positive electrode. The cathode is built from lithium cobalt oxide (LiCoO2). The anode is reared of carbon and both anode and cathodes are suppressed in an electrolyte. The battery is equipped with a separator between cathode and anode then the Lithium-ions from the cathode roams through the electrotype and separator to the anode.
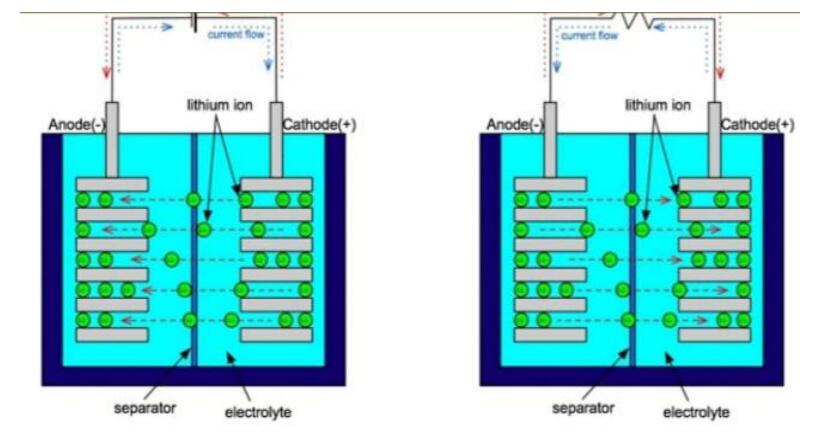
Image 2. Retrieved from physicsandsocietybc.wordpress.com
According to Kejie Zhao's paper on “Fracture Of Electrodes In Lithium-Ion Batteries Caused By Fast Charging.” Journal Of Applied Physics (2010), The electrons from the cathode travel through a metallic wire outside of the battery and amidst this process 3.7 volts are produced in the cell. In its recharging state all of this is reversed and the lithium ions are sent back to the cathode.
Lithium-ion Phosphate
(Read also: Lithium Iron Phosphate VS. Lithium-Ion: Differences and Advantages)
The development dates back to 1996. The University of Texas discovered phosphate as cathode material for rechargeable lithium batteries. Li-phosphate offers good electrochemical performance with low resistance. This is made possible with nano-scale phosphate cathode material (Battery University, 2021). Its fundamental benefits are good thermal stability, extensive safety, tolerance, high current rating and long-term cycle life.
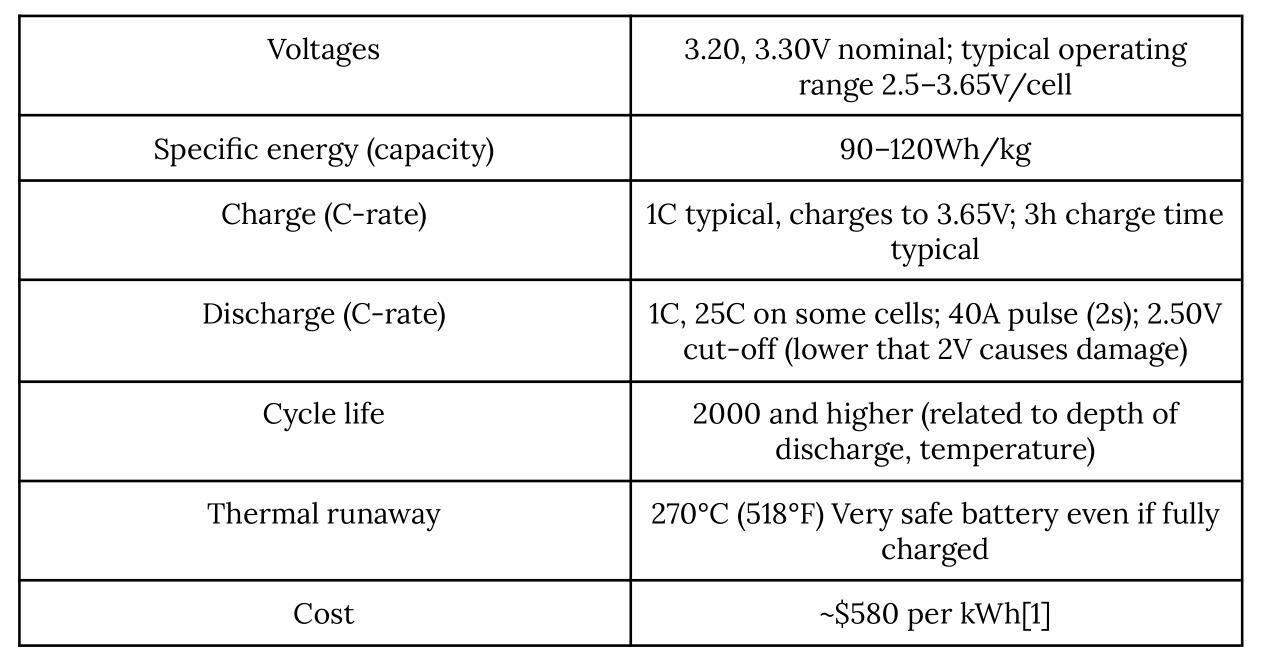
Table 2. Characteristics of Lithium-ion Phosphate (Retrieved from Battery University)
Even when hoarded for a year inside the storage space, Lithium-ion Phosphates batteries remain to have a similar energy density for its slow decline of energy density. Lithium-ion Phosphate batteries are way more dependable and safer than standardized Lithium-ion batteries. It does not decompose at high thermal operations and it is harder to ignite amidst charging and discharging.

评论
发表评论