Batteries for Start Stop Car
Types of Lithium-ion Batteries
Lithium-ion utilizes a cathode (positive terminal), an anode (negative terminal), and an electrolyte as conductors. The anode of a releasing battery is negative and the cathode positive. The cathode is a metal oxide and the anode comprises permeable carbon. During the release, the particles stream from the anode to the cathode through the electrolyte and separator; the charge turns around the bearing, and the particles stream from the cathode to the anode. Figure 1 outlines the cycle.
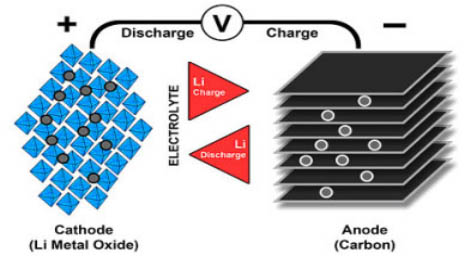
Figure 1: Ion stream in Lithium-ion battery. At the point when the cell charges and releases, particles transport between the cathode (positive terminal) and anode (negative anode). On release, the anode goes through oxidation, or loss of electrons, and the cathode sees a decrease, or an addition of electrons. Charge turns around the development. (Retrieved from Battery University)
Sony's unique lithium-ion battery utilized coke as the anode (coal item). Starting around 1997, most Li-ion makers, including Sony, moved to graphite to achieve a compliment release bend. Graphite is a type of carbon that has long haul cycle soundness and is utilized in lead pencils. It is the most normal carbon material, trailed by hard and delicate carbons. Nanotube carbons have not yet found business use in Li-ion as they will more often than not ensnare and influence execution. A future material that vows to improve the exhibition of Li-ion is graphene. Figure 2 delineates the voltage release bend of a cutting edge Li-ion with graphite anode and the early coke adaptation.
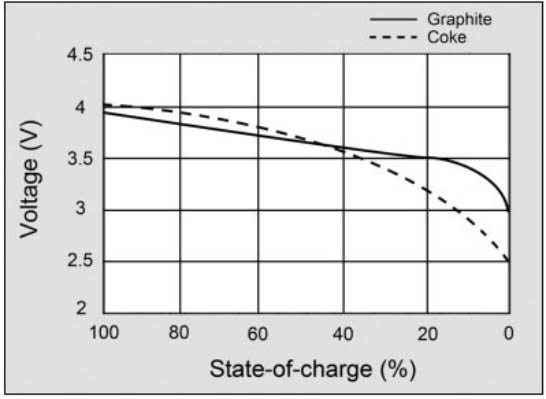
Figure 2: Voltage release bend of lithium-ion. A battery ought to have a level voltage bend in the usable release range. The advanced graphite anode does this better than the early coke variant. Graciousness of Cadex.
A few added substances have had a go at, including silicon-based combinations, to upgrade the exhibition of the graphite anode. It takes six carbon (graphite) iotas to tie to a solitary lithium particle; a solitary silicon molecule can tie to four lithium particles. This implies that the silicon anode could hypothetically store more than 10 times the energy of graphite, however, development of the anode during charge is an issue. Unadulterated silicone anodes are accordingly not down to earth and just 3–5 percent of silicon is normally added to the anode of a silicon-based to accomplish great cycle life.
Utilizing nano-organized lithium-titanate as an anode added substance shows promising cycle life, great burden abilities, fantastic low-temperature execution and predominant wellbeing. However the particular energy is low and the expense is high.
Trying different things with cathode and anode material permits makers to fortify characteristic characteristics, however, one improvement might think twice about. The alleged "Energy Cell" improves the particular energy (ability) to accomplish long runtimes yet at lower explicit power; the "Power Cell" offers remarkable explicit power yet at a lower limit. The "Cross breed Cell" is a trade off and offers a smidgen of both.
Makers can accomplish a high explicit energy and minimal expense generally effectively by adding nickel in lieu of the more costly cobalt. However this makes the cell less steady. While a new business might zero in on high explicit energy and low cost to acquire speedy market acknowledgment, wellbeing and strength can't be compromised. Trustworthy makers place high respectability on wellbeing and life span.
Charging Lithium-ion
Charging and discarding batteries is a substance response, yet Li-ion is professed to be the exemption. Battery researchers talk about energies streaming all through the battery as a feature of particle development among anode and cathode. This case justifies yet on the off chance that the researchers were absolutely correct, then, at that point, the battery would live until the end of time. The fault limit blur on particles getting caught, yet similarly as with all battery frameworks, inside erosion and other degenerative impacts otherwise called parasitic responses on the electrolyte and anodes still assume a part.
The Li-ion charger is a voltage-restricting gadget that has similarities to the lead corrosive framework. The distinctions with Li-ion lie in a higher voltage for every cell, more tight voltage resistances and the shortfall of the stream or float charge at full charge. While lead corrosive offers some adaptability as far as voltage removal, producers of Li-particle cells are extremely severe on the right setting since Li-ion can't acknowledge cheating. The supposed marvel charger that vows to draw out battery life and gain additional limits with heartbeats and different tricks doesn't exist. Li-ion is a "spotless" framework and just takes what it can retain.
Why Opt for Lithium-ion Phosphate?

Lithium iron phosphate batteries (LiFePO4 or LFP) offer lots of benefits compared to lead-acid batteries and other lithium batteries. Longer life span, no maintenance, extremely safe, lightweight, improved discharge and charge efficiency, just to name a few. LiFePO4 batteries are not the cheapest in the market, but due to a long life span and zero maintenance, it’s the best investment you can make over time.
HOUNY has specifically developed and launched a series of car start-stop batteries that use automotive-grade lithium iron phosphate batteries and a built-in intelligent battery management system (BMS).
ABOUT HOUNY
KEEP ON INNOVATING
Visit Website: www.hounypower.com
Telephone: +86 755 8399 4783
Email: market@houny.cn
Mobile: +86 138 2313 0291

评论
发表评论